Quote:
|
Originally Posted by Groth
Just as you can run more current through to increase your depth of anodization, galvanic currents can flow. No matter how non-conductive your aluminum oxide is, andozing produces a porous layer of it.
|
Annodization is a porous layer of aluminum oxide. It is similar to a honeycomb. This is where the dye "attaches itself." It actually collects in the microscopic pores of aluminum oxide.
Quote:
|
Originally Posted by http://www.caswellplating.com/kits/aluminum.htm
At this stage of the process the film has microscopic pores (see picture left) which have to be sealed (or 'fixed') by dipping the part into sealer for 15 minutes. The part may be colored prior to boiling by simply soaking in our range of professional anodizing dyes. An almost infinite range of colors can be produced by mixing the dyes together.
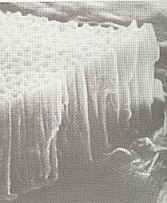 |
Once sealed the product then becomes electrically insulated. Annodizing is a process...one that must be done strictly or poor results will definately be the outcome.
Some places to look for annodizing information:
This site is an incredible sorce of information! And now he even sells Supplies...Great site.
http://www.focuser.com/atm/anodize/anodize99.html
This site has supplies and kits and their book on anodizing is second to none. Very easy to understand.
http://www.caswellplating.com/kits/aluminum.htm
Good source for plating information.
http://www.finishing.com/
The US governments answers to epa regulations of metal finishing products and plating techniques.
http://es.epa.gov/cooperative/topics/metalfin.html
I hope this clears up any misconceptions.
-MC