 | ||
|
||
|
|||||||
| General Liquid/Water Cooling Discussion For discussion about Full Cooling System kits, or general cooling topics. Keep specific cooling items like pumps, radiators, etc... in their specific forums. |
 |
| Thread Tools |
|
|
#26 |
|
CoolingWorks Tech Guy Formerly "Unregistered"
Join Date: Dec 2000
Location: Posts: 2,371.493,106
Posts: 4,440
|
"No DI/DW will not stop corrosion, but it will help to minimize it. High resistivity electrolyte will slow the e-chem process."
this is true, and a stupid recommendation for WCing systems; such water in the presence of mixed metals is ionic in minutes -> why make ineffective recommendations ? (distilled water is worthwhile, but to minimize the contaminants in the system) "Galvanic or disimiler (sp) metal corrosion (al rad and CU block) may be a problem within some peoples WC systems. In most cases there won't be a metallic path so their will be no need for concern." so you are commenting on something that is not an issue ? -> show me a SINGLE instance of such please "A piece of thin zinc ribbon submerged in part of the WC loop (say the res or T line) then connected to the object that is to be protected (the Al rad) would likely provide enough Cathodic Protection to reduce the corrosion rate to a crawl." wrong, and this is a DUMB error you persist in your efforts to demonstrate what ? zinc won't do shit with alu and cu -> what is this "would likely" wording ? if you do not know, shut the lower jaw and stop posting speculation dressed up as fact and the corrosion product of your sacrificial anode ? is that not contamination ? do you think your pump will like it ? -> then don't put it in the system to begin with your resistance to comprehension exceeds my interest, I am abandoning the field |
|
|

|
|
|
#27 |
|
CoolingWorks Tech Guy Formerly "Unregistered"
Join Date: Dec 2000
Location: Posts: 2,371.493,106
Posts: 4,440
|
aiii
missed another ignorant statement "It is also used for protecting burried pipelines, underground storage tanks, sheet pile walls, H piles, and industrial water cooling applications." oh really ? TILT have you heard of impressed current ? Titan151 I worked as a Materials Engineer for Bechtel some 25 yrs ago. The people I worked with were the Corrosion Engineers. There is probably not a great deal you are going to tell me about corrosion and its prevention. |
|
|

|
|
|
#28 |
|
Thermophile
Join Date: May 2001
Location: UK
Posts: 1,064
|
Yeah titan, it's nothing to worry about, just don't come crying here when your cooling system looks a bit like this:
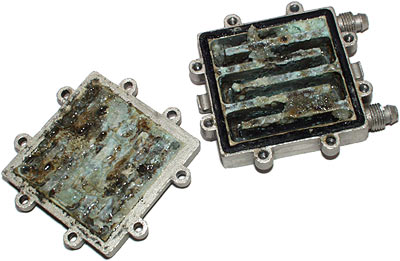 That's galvanic corrosion, from a mixed metal watercooling system. Full write up here: http://www.dansdata.com/burning.htm I suggest you read it, you might find it enlightening.
__________________
Once upon a time, in a land far far away... |
|
|

|
|
|
#29 |
|
Cooling Neophyte
Join Date: Jun 2003
Location: Auckland, New Zealand
Posts: 40
|
you see guys this is the problem ...... no one can actually come up with a definitive answer about how galvanic corrosion works in the real world. all i know is that i had a copper radiator that was not touching the case and when i ran it it killed the aluminium. and with my new systwm with heaps more money spent on it i dont want it to happen
|
|
|

|
|
|
#30 |
|
CoolingWorks Tech Guy Formerly "Unregistered"
Join Date: Dec 2000
Location: Posts: 2,371.493,106
Posts: 4,440
|
wrong, this is all perfectly understood
by people who are appropriately educated and work in the field - that you and your friends do not know is hardly the definition of "the real world" with alu, use an inhibitor - always we have been selling alu components for years w/o problems so long as an inhibitor was used |
|
|

|
|
|
#31 |
|
Cooling Savant
Join Date: Apr 2004
Location: Seattle
Posts: 116
|
None of you are understanding what I am saying. The original statement bought up in this thread was about corrosion concerns between an Al rad and a Cu block. I simply stated that their will be no corrosion cell between the Al rad and the Cu block if there is no metallic path.
As for corrosion issues within a single object, such as a radiator, that is a different story. Here the metallic path cannot be cut and the cell will set up between dissimilar segments of the same metal. You can minimize corrosion by using DW or DI water. To what extent this water remains high in resistivity is a reasonable question. I think I will take a sample of water out of my water cooling loop and measure the resistivity. It will be interesting to see how much it has changed since I first put it in. Perhaps the best option is to use metals that are either galvanized or anodized. This will also help to minimize the problem in the first place. Also, the use of Cathodic Protection is extremely common and any many industrial apps. I will set up a system and test it for anyone interested. I guess I should add that I am a Corrosion Engineer by profession. This doesn't make me right, it's just that I deal with this type of thing every day. Sometimes in explaining things I forget to describe things that to me are common knowledge. So when I talk about the corrosion cell between the rad and block I am not saying anything about the corrosion cell that may be present with the rad itself. That's all I have to say. If someone would like to know more about this topic please pm me as it isn't worth posting on these forums.
__________________
Water Cooled AMD XP 4200 X2 Asus A8N-SLI Twin Raptors in Raid 0 Configuration 2 Gigs Corsair 3200 7800 GTX |
|
|

|
|
|
#32 |
|
CoolingWorks Tech Guy Formerly "Unregistered"
Join Date: Dec 2000
Location: Posts: 2,371.493,106
Posts: 4,440
|
good description
why could we have not started here ? sorry Titan151 |
|
|

|
|
|
#33 |
|
Cooling Neophyte
Join Date: Jun 2003
Location: Auckland, New Zealand
Posts: 40
|
not that it matters anymore but i bought a copper car radiator instead of the Al one. now i just need to figure out how to get all the tust /stuff out of it ( clean the inside ) what do most people do ?
|
|
|

|
|
|
#34 |
|
Thermophile
Join Date: May 2001
Location: UK
Posts: 1,064
|
I used a CLR flush - seemed to get most of the crap out (hard to tell for sure without hacking open the rad).
__________________
Once upon a time, in a land far far away... |
|
|

|
|
|
#35 |
|
Cooling Savant
Join Date: Jan 2003
Location: BC, Canada
Posts: 313
|
Swish diluted acetic acid plus a little sodium in it - the copper will be clean and bright in seconds. Rinse. Yes, that's just vinegar and a dash of salt, and extra water if you're really cheap.
Boiling it in a covered oven roasting pan will free any oils trapped in there. |
|
|

|
|
|
#36 | |
|
Cooling Neophyte
Join Date: Feb 2004
Location: Singapore
Posts: 67
|
Quote:
 sodium chloride will do though |
|
|
|

|
|
|
#37 |
|
Thermophile
Join Date: May 2001
Location: UK
Posts: 1,064
|
I remember sodium into water at school, it caused a nice satisfying explosion.

__________________
Once upon a time, in a land far far away... |
|
|

|
|
|
#38 | |
|
Cooling Savant
Join Date: Jan 2003
Location: BC, Canada
Posts: 313
|
Quote:
 Thank you. Now excuse me while I go wander into traffic. Thank you. Now excuse me while I go wander into traffic.
|
|
|
|

|
 |
«
Previous Thread
|
Next Thread
»
| Currently Active Users Viewing This Thread: 1 (0 members and 1 guests) | |
|
|
All times are GMT -5. The time now is 07:36 AM.












